4 ways to help reduce surgical site infections
Surgical site infections (SSI) are on the rise. What’s your plan to reduce SSI? Consider these four tips before you operate:
1 | Begin with the skin
2 | Tables take cover
3 | Beware the colonies
4 | Dress for success
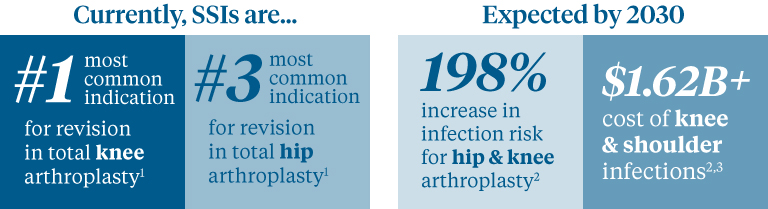
Whether you’re doing total joint procedures today or plan to, it’s important for your staff to have the tools and knowledge to help prevent, diagnose and treat surgical site infections (SSI).
1 | Begin with the skin
About 80% of skin flora occurs on the outside layers of the skin.1In fact, one square centimeter of skin can host as many as 10 million aerobic bacteria, a leading cause of healthcare-acquired infections.2
Try a single-use skin prep applicator to help reduce skin flora. For example,BD’s ChloraPrep® Prep Solution with Tintmoves the clinician’s hand away from the patient’s skin for a more aseptic technique.* The proprietary tinting process also allows the clinician to see the prepped area more clearly.
2 | Tables take cover
The Association of periOperative Registered Nurses (AORN) guidelines state that the sterile field – including tables – should be covered if not being used immediately.6You can help significantly reduce the risk for contamination by covering even just portions of the sterile field that are not in active use.6
The American Journal of Infection Control recently published a study stating that covering sterile tables reduced bacterial contaminations at four and eight hours. The study also suggested that covered tables reduce the amount of bioburden that can collect on unused instruments.7
Are you covering your tables and stands? These newback table coversandmayo stand coversmake it easy.
3 | Beware the colonies
Research suggests risk of SSI increases up to nine times due to nasal colonization of Staphylococcus aureus, presenting a big challenge in surgical settings.8
30% of people are already nasally colonized with S. aureus when they reach the OR.8
S. aureus, also known as MRSA, is the leading cause of SSI, and yet surgical settings don’t always include testing for this bacteria in their pre-op care.
Nasal swab tests allow you to detect and identify MRSA for better prevention and control.Scalable instrument modelsfit into a wide variety of testing environments, including surgery centers that are usually challenged for space.
You can trace 80% of wound infections to the patients’ own nasal flora.9
Proactive clinicians have modified their pre-op protocol to treat every surgical patient with a nasal iodine-saturated swab, often with favorable results.10For example, the Portland VA eliminated SSI completely when they began swabbing every patient with povidone iodine two hours before surgery.9
In response to an uptick in S. aureus infections among total joint patients, TRIA Orthopedic Center in Bloomington, MN started a screening program that brought infections to a halt. Lori Groven, MSPHN, RN, CIC and infection preventionist with TRIA suggests placing an order for pre-op screenings as soon as the surgery is scheduled.10
Try easy-to-use PVP-iodine nasal swabsticks for a compelling long-term cost benefit.PDI Healthcare’s Profend™can kill 99.7% of S. aureus at one hour and 99.9% at twelve hours, and takes just 60 seconds to apply, according to PDI.
4 | Dress for success
Not all orthopedic surgical gowns are created equal. When selecting a gown, be sure to choose one that’s resistant to tears, punctures, strikethrough and fiber strains.
High-quality surgical gowns should meet or exceed testing standards of the American National Standards Institute and the Association for the Advancement of Medical Instrumentation and should be certified to meet AAMI level 3 standards for all critical zones.
These gowns, for example, meet these rigorous standards and feature a cool-back panel for maximum comfort.
1 | READMISSION RATES, CAUSES, AND COSTS FOLLOWING TOTAL JOINT ARTHROPLASTY IN US MEDICARE POPULATION W. Murphy, P. Lane, B. Lin, T. Cheng, D. Terry, S. Murphy; Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468 (1):45-51.
2 | Kurtz SM, Lau E,Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8) (suppl):61-5.e1.
3 | Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785
4 | Brown E, Wenzel RP, Hendley JO. Exploration of the microbial anatomy of normal human skin by using plasmid profiles of coagulasenegative staphylococci: search for the reservoir of resident skin flora. J Infect Dis. 1989;160(4):644-650.
5 | Hibbard JS. Analyses comparing the antimicrobial activity and safety of current antiseptic agents: a review. J Infus Nurs. 2005;28(3):194-207; Sievert D. Antimicrobial-resistant pathogens associated with healthcare associated infections: summary of data reported to the NHSN at the CDC, 2009-2010. Infect Control Hosp Epidemiol. 2013;34(1):1-14.
6 | AORN Guidelines for Perioperative Practice, 2018 Edition
7 | Markel, et al. Covering the instrument table decreases bioburden: An evaluation of environmental quality indicators, American Journal of Infection Control, 2018
8 | Price CS, Williams A, Philips G, Dayton M, Smith W, Morgan S. Staphylococcus aureus nasal colonization in preoperative orthopaedic outpatients. Clin Orthop Relat Res. 2008;466(11):2842-2847
9 | Melissa S. Schmidt, MSN, RN, CNL, CPAN, CAPA, PACU.; VandenBergh MF, Yzerman EP, van Belkum A, Boelens HA, Sijmons M, Verbrugh HA. Follow-up of Staphylococcus aureus nasal carriage after 8 years: redefining the persistent carrier state. J Clin Microbiol. 1999;37:3133-3140
10 | Outpatient Surgery, 2018
*Supported by clinical evidence from more than 50 peer-reviewed publications. Application process does not apply to swabstick format.
所有商标和注册商标是property of their respective owners.
Be advised that information contained herein is intended to serve as a useful reference for informational purposes only and is not complete clinical information. This information is intended for use only by competent healthcare professionals exercising judgment in providing care. McKesson cannot be held responsible for the continued currency of or for any errors or omissions in the information.
The product information contained in this document, including the product images and additional product materials, was collected from various supplier sources. All product claims and specifications are those of the product suppliers, not McKesson Medical-Surgical or its affiliates (“McKesson”) and have not been independently verified by McKesson. McKesson is not responsible for errors or omissions in the product information.
The properties of a product may change or be inaccurate following the posting or printing of the product information in the document, either in the print or online version. Caution should be exercised when using or purchasing any products from McKesson’s online or print documents by closely examining the product packaging and the labeling prior to use.
由于产品变化,信息列出document is subject to change without notice. This information is placed solely for your convenience in ordering and McKesson disclaims all responsibility for its completeness and accuracy, whether or not the inaccuracy or incompleteness is due to fault or error by McKesson.
© 2019 McKesson Medical-Surgical Inc.